News
January 31, 2024
Mitsubishi Materials Corporation
Yokohama National University
Developed a New Titanium Electrode with a Double-Layer Structure Using 3D Printer Technology
- Novel Material That Supports Efficient Hydrogen Production At High Current Densities -
In a joint research and development project, Mitsubishi Materials Corporation ("MMC") and Yokohama National University's Shigenori Mitsushima (Professor at the Faculty of Engineering and Director of the Advanced Chemical Energy Research Center, Institute of Advanced Sciences) and his colleagues have developed a new titanium water electrolysis electrode capable of operating with high efficiency even under high current density conditions.
For realization of a decarbonized society, hydrogen is in high demand as a clean energy source that does not generate carbon dioxide. One hydrogen production technology that is attracting attention is polymer electrolyte membrane (PEM) water electrolysis, an efficient hydrogen production technology with low environmental impact. This electrolysis technology can produce highly pure hydrogen with pure water at temperatures below 100°C and electricity. However, the system cost is high, and this has generated a need to reduce the consumption of precious metal catalysts such as iridium oxide that have a high cost burden.
Against this background, Yokohama National University, which has been entrusted with the Advancement of Hydrogen Technologies and Utilization Project by the NEDO (*1) and has cutting-edge electrode evaluation technology, and MMC, which has highly complex titanium material sintering technology, worked on the development of a new water electrolysis electrode made from titanium.
- (*1)
- New Energy and Industrial Technology Development Organization
Using an evaluation technique that analyzes the performance of an electrolytic cell based on the division into different factors such as the resistance polarization of the electrolyte membrane, activation polarization of the electrode catalyst and the current collecting resistance and diffusion overpotential of the electrode, which is a result of the NEDO project, we have found that an elaborate double-layer structure with different functions is effective in improving the efficiency of water electrolysis electrodes. However, electrodes consisting of two layers with different structures cannot be manufactured in an integrated manner by conventional manufacturing methods because the scale of spatial design required for each electrode layer is different. Accordingly, MMC adopted a binder-jet (*2) 3D printer, which offers a high resolution and degree of freedom, and conducted research and development on the new titanium sintering technology needed for manufacturing elaborate double-layer electrodes, enabling the manufacturing of electrodes with a double-layer structure.
- (*2)
- Method of manufacturing parts by laminating a thin spread of powder while coating it with a binder, solidifying it as a compact in a drying oven and sintering it
By utilizing this newly developed titanium electrode for water electrolysis, a water electrolysis system can be operated with high efficiency even under high current density conditions. It also contributes to the reduction of hydrogen production costs by reducing the consumption of materials such as precious metal catalysts. In the future, we will continue to develop and improve electrode structures for practical use.
[Overview]
Features of the newly developed titanium electrode for water electrolysis
- Applying our original powder sintering technology of pure titanium to 3D printer technology, we achieved the formation of newly doble-layer structures that has a water-splitting part and a diffusion part for oxygen discharge.
- Forming a double-layer structure integrating a layer that comes into contact with the catalyst layer over a wide area to increase the catalyst utilization ratio and a layer that efficiently discharges oxygen generated by the water electrolysis reaction allows reduction of the residual oxygen gas generated inside the electrode.
- With a unique discharge path provided for oxygen bubbles after the electrolysis, the rise of diffusion overpotential can be suppressed even at a high current density of 4 A/cm2 or more. It also serves as a channel mechanism for supplying water to the reaction section and the electrolysis can be performed at high current density with this electrode alone.
- The optimum electrode structure suitable for the electrolytic cell can be manufactured.
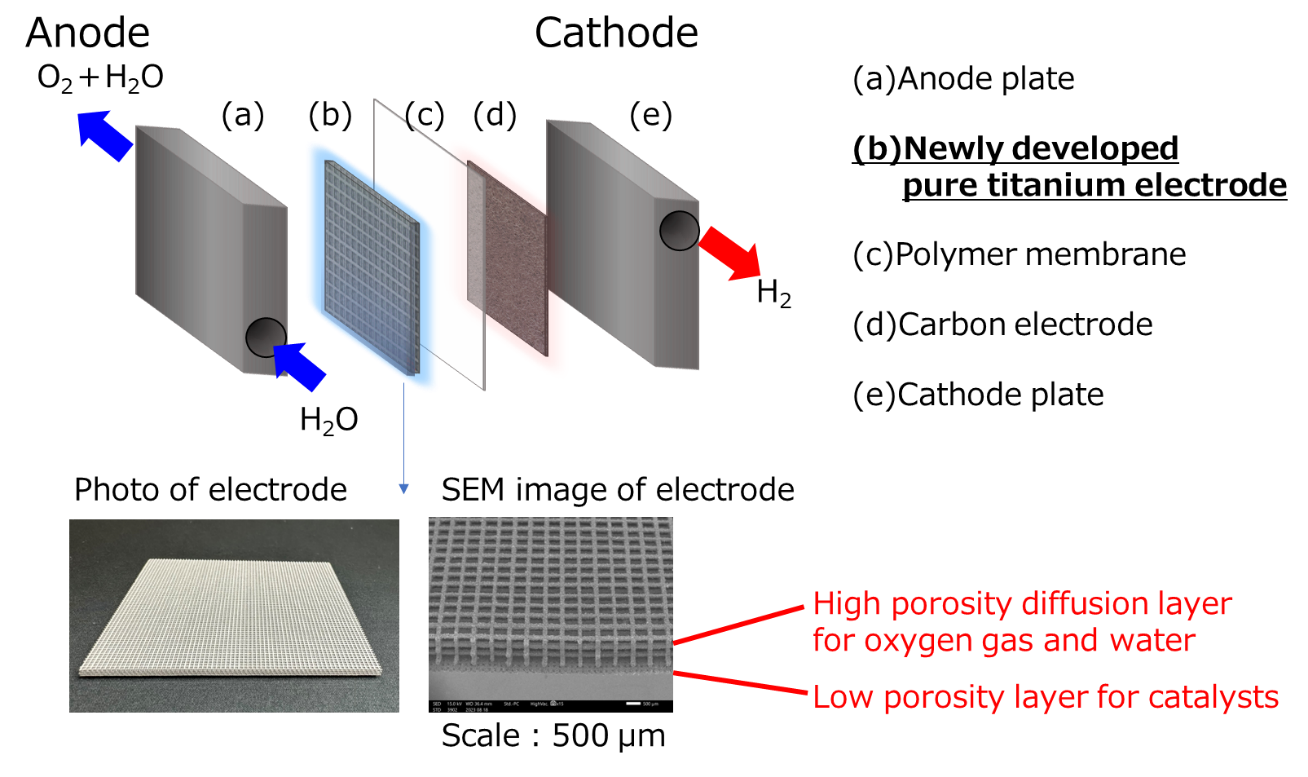 Schematic diagram of PEM water electrolysis
Schematic diagram of PEM water electrolysis
<Contact details for inquiries>
Mitsubishi Materials Corporation:
Corporate Communications Dept., Strategic Headquarters:
+81-3-5252-5206
Yokohama National University:
General Planning Dept., Public Relations and Endowment Division:
E-mail: press@ynu.ac.jp